Hot Products
where do we get metals from and how do we get pure metals?
E-mail: market@hxjq.comnow, if i ask you where do we get the metals from? metals like sodium, potassium, gold, silver and so on. where do we get these metals from? and how do we get the pure metals? let's see.
1: What's the difference between minerals and ores?
we have seen the metal activity series. The metals on the top are very reactive and the bottom are the least reactive. the least reactive exist in the native state and for the reactive metals, they always occur in the form of compounds. they combine with other elements and they always occur in the form of compounds. so, minerals are the natually occuring compounds of metals which are associated with certain substances like soil, sand, etc. so, these are the natually occuring form the metals in the form of compounds.
if your garden soil contains iron, so, soil is a mineral for iron. but can you extract this iron from garden soil? no, you cannot, there are a lot of impurities and it is very difficult to extract iron from this garden soil. so, the minerals, the particular minerals from which we can extract the metals commercially, at a low cost and with minimum effort are known as ores.
for example, limestone is an ore of calcium, we can extract calcium metal from this ore limestone. similarly, bauxite, this is an ore for aluminium. Aluminium is extracted commercially from the bauxite ore.


so, based on the reactivity series, we know that the metals on top, they never exist in the native state. they are very reactive. they combine with other elements to form the ores and from these ores we can extract the metals. the metals at the bottom of the series, since they are very less reactive, they mostly occur in the native state, for example gold, silver, platinum, they are least reactive and so they are found in the native state in the nature.
2: how to extract the metals from the ores?
so, now we have the ores, our next step is to extract the metals from the ores. so, we have to carry out the extraction of metals from the ores. the first step in this is crushing or grinding the ore that we get. so, at the industrial level, there are big machines which crush and grind the ore by stone crusher and ball mill.

next step is to concentrate the ore, after crushing and grinding, there are still some sand and soil particles which are attached to the ore. these soil and sand particles are the impurities of the ore. so, now we have to purify this ore that is we have to concentrate the ore, we have to remove these impurities like sand and these impurities which are associated with the ore are known as gangue. so, the impurities associated with the ores are known as gangue and we have to remove these impurities in order to purify the ore. so, now we have to concentrate the ore.
3: How to purify the ore?
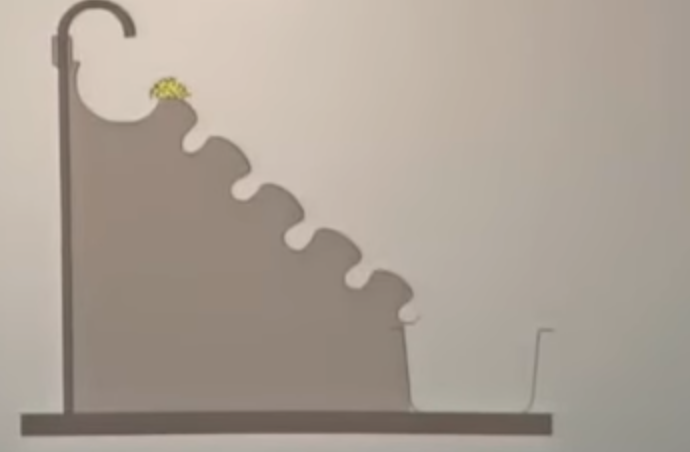
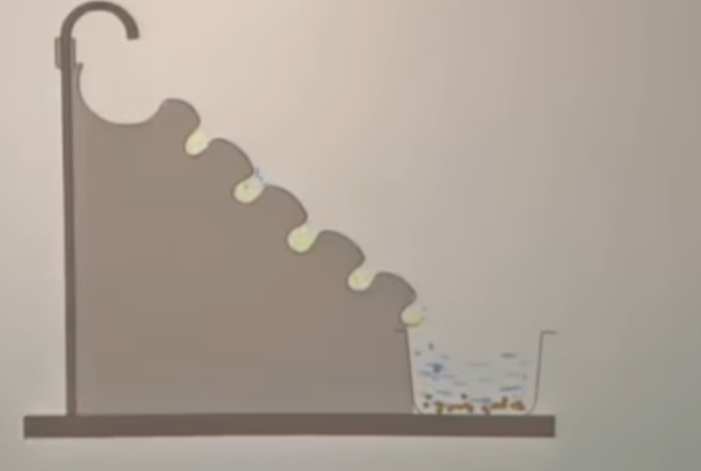
that is how do we concentrate the ore and remove these gangue particles. there are different methods to carry out the concentration of ore. the first method is by using the hydrolytic method. hydro means we have to use water in this method and it is also known as gravity separation. so from the gravity separation, we know that it uses the difference in the densities between the ore and the impurities.
so, this is the setup for the method of hydrolytic separation. in this , the ore is placed on the top, this wall has grooves inside it. so, now we will pass a jet of water, the impurities like sand etc, they are very light. so, when we pass water- a jet of water the light impurites can be carried away and the heavier ore particles, they settle in these grooves. so, the impurites being ligheter, they are washed away and the denser ore particles they settle inside the grooves.
- NEXT: End!
- PREV: Small Ball Mill of 2t/h for Gold Ore






